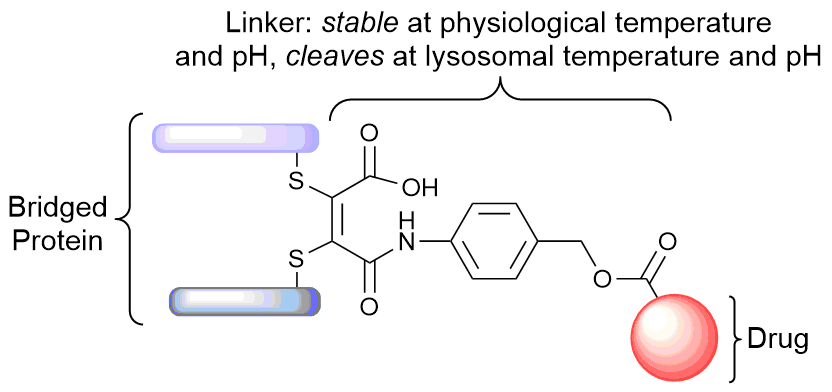
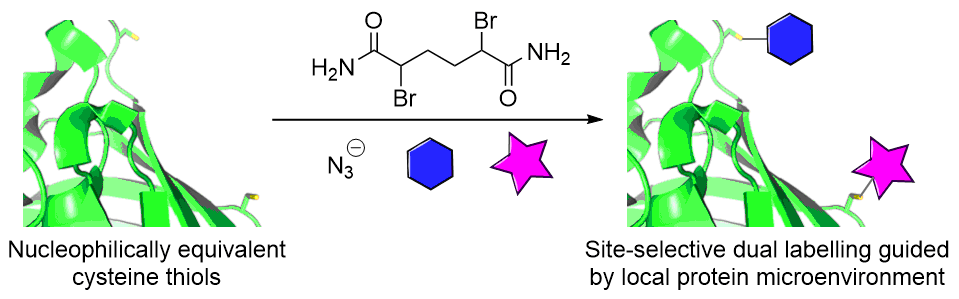
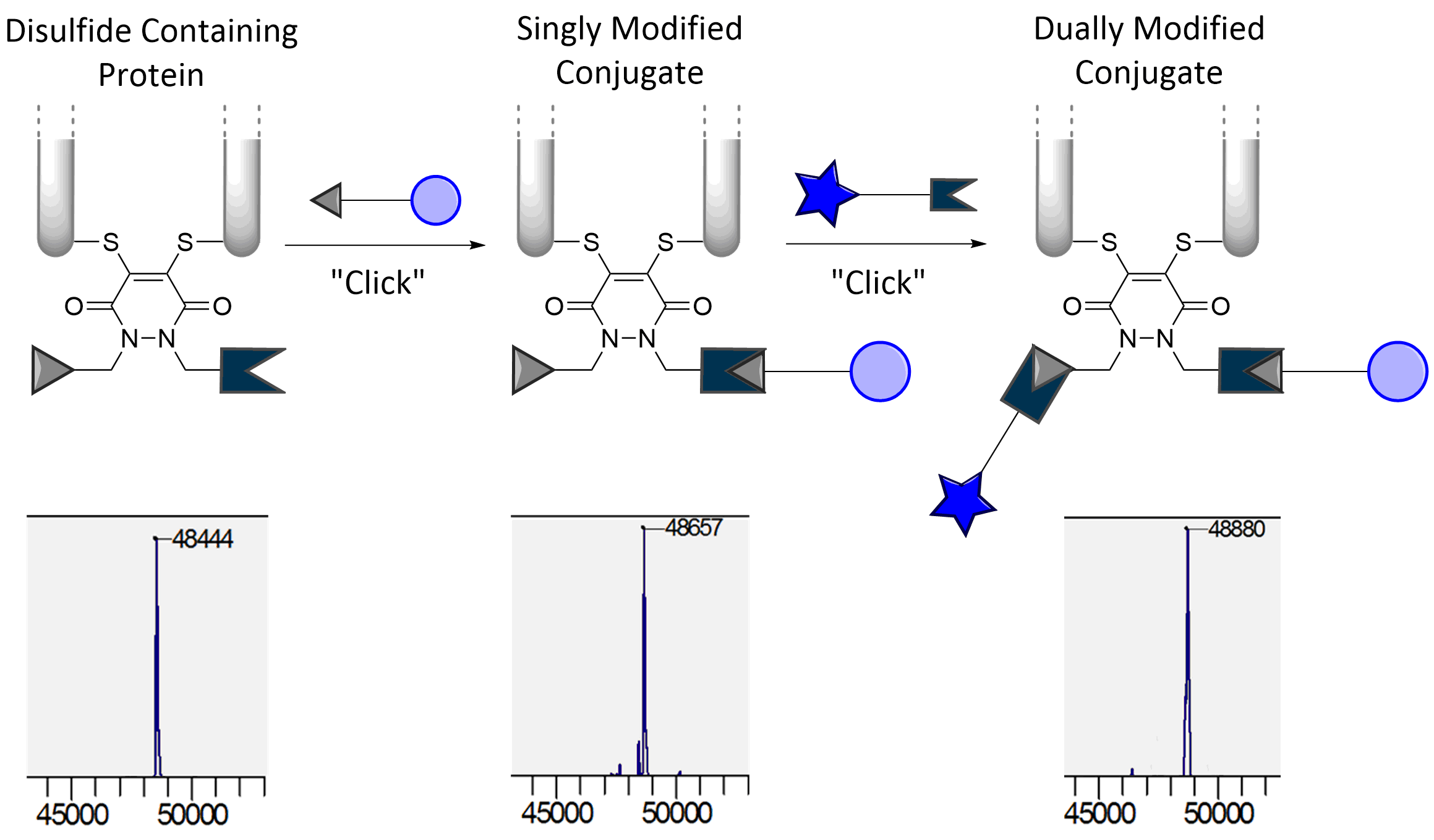
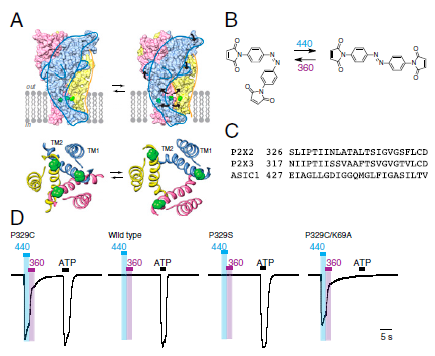
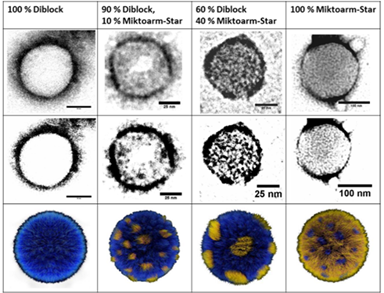
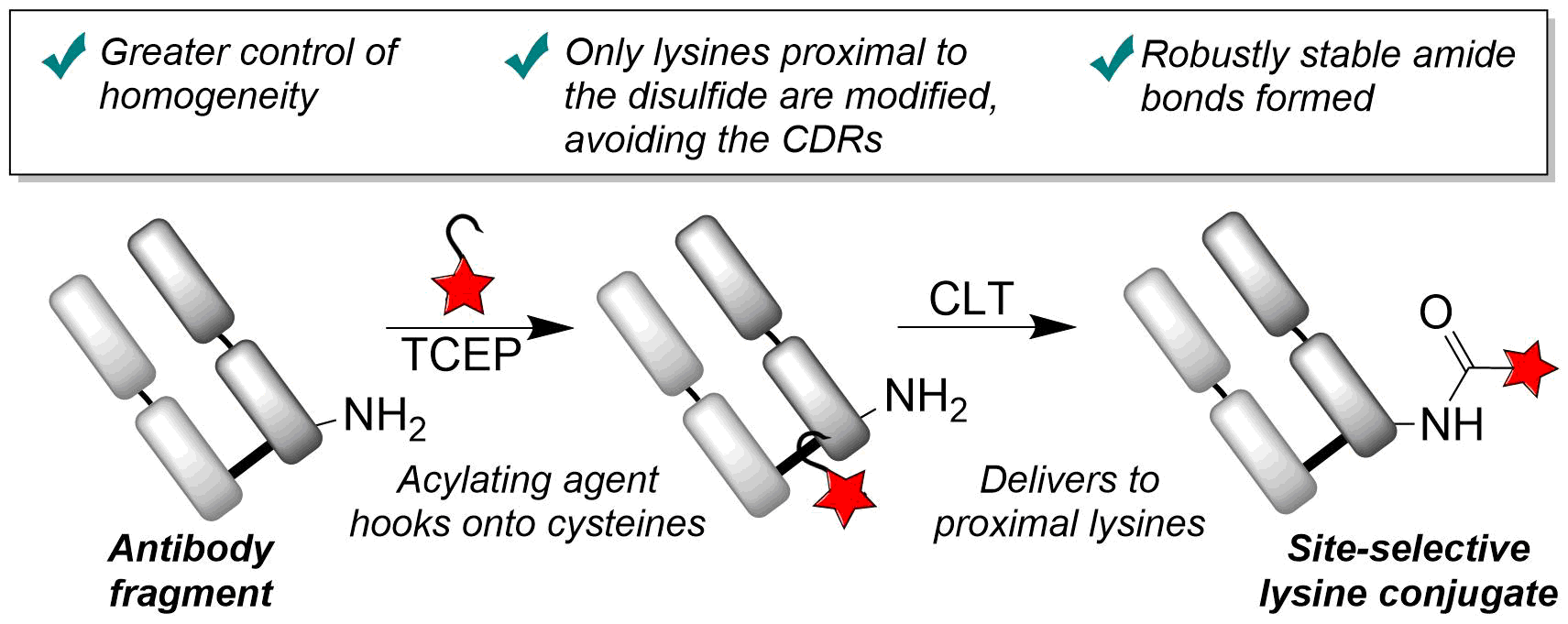
Our group is primarily focused on answering important biological questions through the application of Organic Chemistry. We do this by designing and tuning a variety of chemical tools (from chemoselective reagents with orthogonal “click” handles to photoswitchable azobenzenes) for the particular system we are studying.
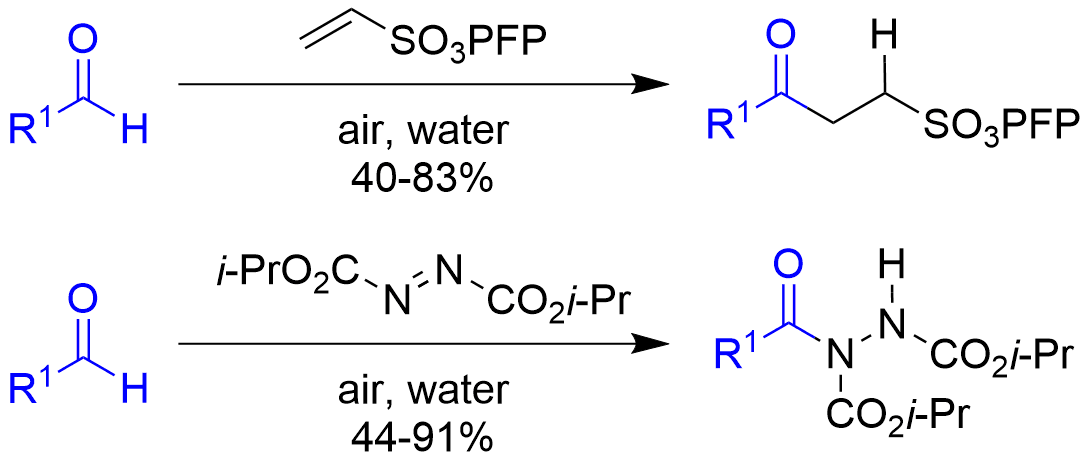
We also work on novel methodologies based on aerobic C-H activation for the functionalisation of aldehydes, azo-dicarboxylates and various alkenes in a controlled manner.